Probiotics as Targeted Antibiotics
by
The rise of public awareness of the bacteria living in our guts has occurred rapidly enough that it seems only a few years ago that probiotics were not much more than yoghurt drinks for your stomach, and when asked if they work, the typical response was a happy-go-lucky ‘Erm, not sure. Maybe … Well, they taste good! I have one at breakfast!’
Well ‘having one at breakfast’ was about the extent of the incentive for why people continued to purchase them, but fortunately, probiotics have come a long way since then.
Let me paint a picture of where probiotic supplements could go next. This will sound familiar to a lot of you who’ve given consideration to this and been following the topic. It makes a tonne of sense on paper, and you’ll be nodding to yourself, saying ‘Yup, this is what we’re after. Now we wait!’ Then, I’ll explain why I think that path is not the next step for probiotics and where I believe that it will go instead, in the shorter term (i.e., the direction I am pursuing with Elixa). Both ideas make sense and will have portions of truth in them.
The first scenario goes like this:
Whatever problem we have (of gut origin) is due to a lack of specific useful microorganisms. The future of probiotics is about offering the exact species; nay, the exact strains that will supplement those gaps. They will then colonise their niche in the gut and plug up the missing portion of a strong microbiotic profile. Sort of like curing a vitamin deficiency.
In this scenario, the way forward for probiotics is identifying these strains that are commonly deficient in the modern human. This could either be presented as different blends for different conditions or deficiencies: analogous to taking just Vitamin B6 or just Zinc, etc.
Or as an all-in-one formula tailored to the statistical average: analogous to taking a multivitamin containing RDA amounts.
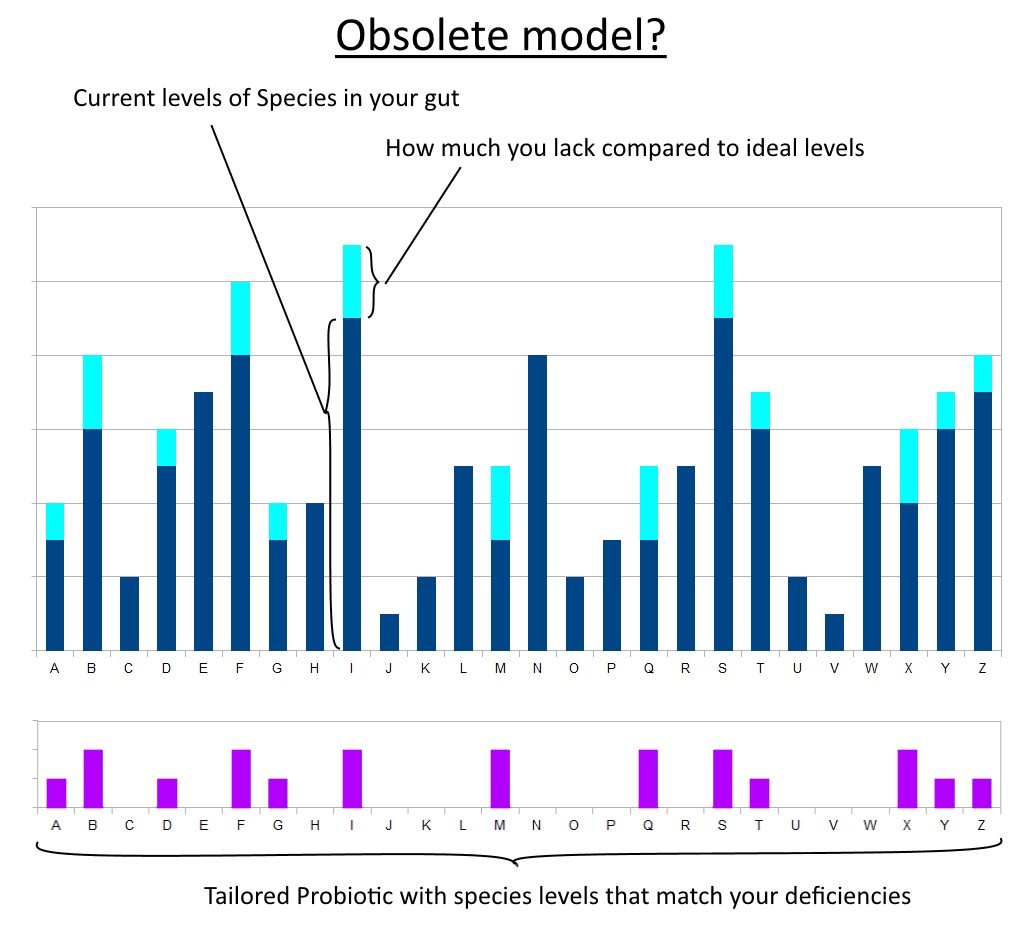
‘Good Day Mrs. Health Store Owner. I’m found to be lacking in Bacteroides Thetaiotaomicron.’ (She consults her alphabetized shelf full of probiotics)
‘Ah yes, we have just the thing.’ (Hands you a bottle of capsules filled with that species.)
Ideally, you could even use some quantitative method to assay your microbiome beforehand. Thus being able to feed in this data and have a personalised blend for your needs.
Sounds good and a lot of truth in it, perhaps.
A slightly different take on this would be a probiotic that didn’t superimpose onto your native microbiota to make up for deficiencies, but moreover replaced large portions of microbiota. Think of the first scenario as pasting text into select places in a paragraph and the second scenario as pasting it with the current paragraph highlighted, i.e. replacing the entire paragraph of highlighted text with a better paragraph. The ultimate form of this would be a probiotic equivalent of a fecal microbial transplant (“FMT”) from a pure gut, whereby some think the aim is to replace the entire microbiota with a superior profile of microbes.
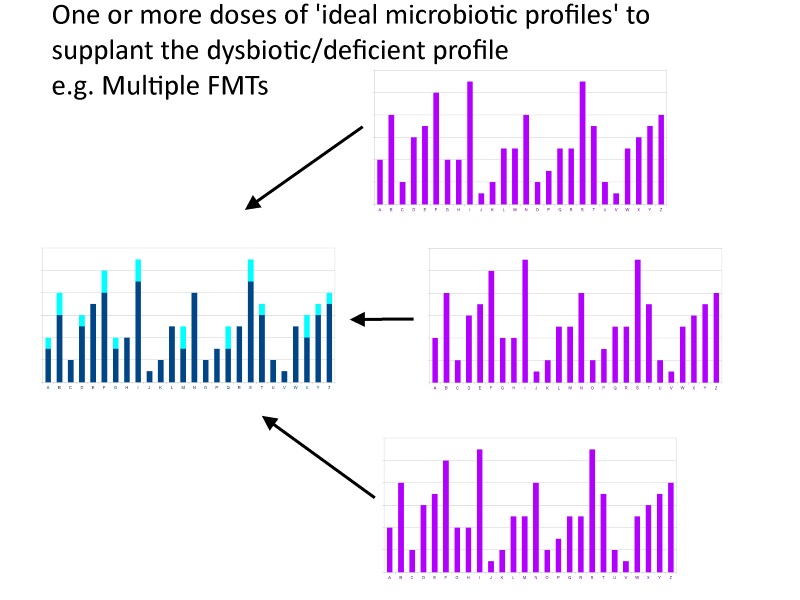
I don’t disagree with these scenarios in any sensationalistic, ‘complete baloney!!’, kind of way. And I think the probiotic equivalent of an FMT is going to be the quintessential probiotic, eventually. Just not in the short term. And I don’t think the supplementation model is going to be the intermediate stepping stone, nor do I think the current FMTs work via the mechanism of supplementing deficiencies.
I base my current R&D strategy on the hunch that probiotics’ fundamental mode of operation is different.
Preface
First, I should explain a hunch I have that may differ from the norm.
The profile of the flora in our guts is assumed to be the cause of the majority of gut-related conditions. I should mention that there are other gut attributes to be considered, such as gut hyperpermeability or damage and inflammation to the gut lining causing, for example, malnutrition. But let’s go back to the gut flora (aka. the microbiota) because I think it’s likely that it is the main culprit and is linked to the other two items. The microbiota is a diverse community of microorganisms within our large intestine. They’re all bugs, but the taxonomic differences between some of these bugs are greater than the gap between you and your pet dog (and far more exotic organisms).
We assign most of the blame for gut-related conditions to a lack of some species in your gut. E.g., ‘I am deficient in Akkermansia Muciniphila; therefore, I am at greater risk of becoming obese from a similar diet to someone who is not deficient.’
Or, a more general example: ‘I have eczema due to lack of bacteria X.’
I think that the cause of the vast majority of these conditions is not a lack of good species, but instead an excess of harmful bacteria.
‘Huh, what’s the difference? Too much bad, not enough good … imbalance … balance … we need to ‘balance’ the gut …. the same thing right?’
The difference is simple but fundamental: It’s not condition Y due to lack of bacteria X. It’s condition Y due to the presence of non-beneficial bacteria Z in my opinion, in the majority, but not all, of cases.
Why do I think that? Mostly based on one observation.
People who undergo a total colectomy do not suddenly develop all these myriad conditions that result from a dysbiosis. If everyone who had their (entire) large intestine removed, suddenly developed depression, dermatitis, acne, diabetes, allergies, etc., then it would be common knowledge!
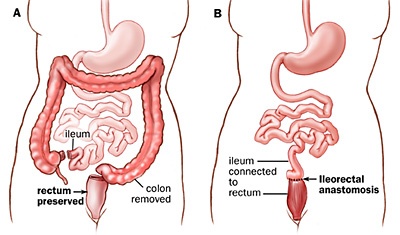
Total and subtotal colectomies can indeed wreak havoc on the functional capability of the digestive tract, but they do not cause all these conditions of dysbiosis to present.
Without a large intestine (total colectomy) you don’t have any large-intestine bacteria because you don’t have a large intestine. So if a deficiency in beneficial species were the cause of the majority of symptoms of dysbiosis, then a total colectomy would result in the acquisition of the majority of problems (benign and severe) from the entire spectrum of dysbiosis-related conditions.
It’s simple logic that you can mull over yourself and make up your mind on your own. Don’t assume I’m right. That’s why I explained it all. So you can pick it apart. Think about it and let me know if I’m making a glaring error. To play devil’s advocate: Perhaps the Ileum undergoes significant adaptation and takes up some of the microbiotic ‘slack’?
Beneficial bacteria are clearly useful for something – extremely helpful, in my opinion. Both directly and indirectly. They play vital roles, and a sterile gut is not a healthy gut. They are the best defence from acquiring an overgrowth of other harmful microbes. That alone is worth their existence. Plenty of rodent experiments have demonstrated the serious consequences of having a sterile gut. They use Gnotobiotic rodents. Gnotobiotic means known life, i.e. the microbiota is fully known to the scientist. In this case, it is known to be non-existent – there’s no gut flora whatsoever. This can be achieved by using antibiotics from birth and keeping the rodent in a sterile environment.
They develop problems. Behavioural and immune and more. This demonstrates that good bacteria do play a role in maintaining a healthy organism. However, it also proves that lack of good bacteria is not responsible for the majority of dysbiotic conditions. Otherwise, the rodents would develop the majority of all medical conditions linked to gut dysbiosis. Not just a few.
Probiotics as targeted antibiotics
Mainstream awareness of probiotic supplements (post-yoghurt era) began a little like this:
(Cue sarcasm)
So you’re looking through the list of ingredients on the plethora of probiotics available online and on the shelves, and you recognise some names of species that featured in a study or an article, and you recall that this species was an important one which you would do well to supplement with, because it is very likely that you’re deficient in that one and it’s a good’un after all. At least according to the article and study you recall. What was the outcome of the study? Not really sure but it was studied, and the study was a science one. I read the first line of the Abstract, and it probably went on to conclude something very significant otherwise it wouldn’t have been a study, now would it?

With so many bacterial genera and species in our gut, it seems handy that there are these golden ones which are readily available in a supplement.
Take Lactobacillus Acidophilus. Good old Acidophilus. The tried and tested old-timer whom everyone knows is a beneficial species. Never lets us down. It’s in all the probiotics, and it’s likely one of the very first species of probiotic you ever heard about.
Don’t ask why it’s good. Everyone knows. No need to ask. Everyone’s heard of it. And look – here’s Lactobacillus Casei. Oh boy, it has the same L-word so it must be another home run!
Then things get advanced. Now there’s this new kid on the block – It’s called Bifidobacterium. Wow. Doesn’t even have Lactobacillus in the name. Variety … yeah, I like that. Variety is good. It means we fill a greater variety of deficiencies. GREAT!
Then probiotics with 5 strains and 6 strains and 10 strains (and so on) came out. Wow. That’s a wide spectrum probiotic right there! That’s the profile I want my gut to be. If I take enough of this probiotic, my gut will have this perfect ratio of these potent probiotic species. The special ones. No room for the non-special ones that I’ve not heard of.
(/Sarcasm)
The reality (as I’m sure many reading this blog are aware) is that there are tonnes of species in the large intestine. And the majority of probiotics currently available only contain a handful of species from two genera! And guess what – Lactobacillus isn’t that special! It’s no coincidence that it is straightforward to manufacture and has been churned out by the bucketload due to the dairy industry, where it’s used as a starter culture.
Ok, so I take that back. It is special because it is a beneficial genus which we can manufacture right now. A decent probiotic that is available is much better than an amazing probiotic that is not commercially available – obviously.
Bifidobacterium is a little bit different. It’s towards the ‘more beneficial but harder to culture’ side of the spectrum.
So why all this talk about Lactobacillus? Why all the studies focused on that genus? Well, it’s simple. You may think that a microbiologist established the healthy species of bacteria necessary for optimal human health and then went about manufacturing them. However, it was somewhat the reverse of that: What was sold was determined by what was possible to manufacture – already established by existing industries. The demand was stimulated in a targeted manner based on what is available to sell. There’s no use promoting the benefits of some species which we can’t even put in a pill yet. But I don’t think there’s anything conspiratorial about this. It’s just a case of dealing with what’s to hand versus waiting until perfection has been achieved. Let’s leave the ‘big pharma’ conspiracies at the door and appreciate that industry got things rolling in the first place.
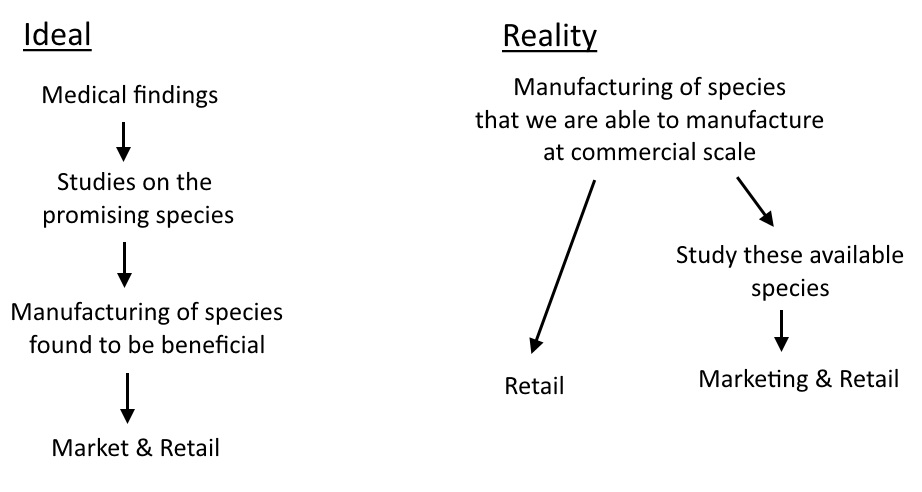
Since the mainstream understanding of probiotics was molded by the contemporaneous technology and understanding, it means a little bit of unlearning may be required. Firstly: I do not believe that the species in Elixa are ones that everyone is ‘deficient’ in. And I do not think the ratios of the formula are proportional to the desired ratios in the gut. And I do not think that it is designed to supplant your pre-existing flora and shift the gut towards a pitifully non-diverse, 12-species, 2 genus profile!
So if it’s not working to plug up the deficiencies in select species, then what’s it doing?
This is the point of this article.
I think that the primary mechanism by which probiotics work is better thought of as targeted (intra-luminal / non-systemic) antibiotics. NOT as a multi-vitamin that plugs deficiencies.
Let’s look at what I mean by this.
‘Intra-luminal / non-systemic’ – Ok, this is pretty simple so forgive me for I have, at the least, used unnecessarily long words. It just means a substance whose direct action takes place within the gastrointestinal tract and does not enter the bloodstream. The only reason to make this distinction is because most antibiotics enter the bloodstream. Simple enough.
Antibiotic – In this analogy, it is referring to the capacity to destroy microbial populations. Simple.
Targeted – This would be the holy grail of antibiotics. The ability to target only bad species and ignore the good ones. Well … guess what – beneficial gut bacteria do exactly that. They have adapted to do so during the millions of years they have co-existed within mammalian guts. If they treated other beneficial bacteria in the same way that they treated non-beneficial bacteria, then the gut would always revert to one (or 2-3, if separated by significant distance) dominant species / genus / strains in the gut. And if it were not true that they counteract the proliferation of non-beneficial bacteria then all the evidence for competitive exclusion would not exist (i.e., the ability of a gut filled with healthy bacteria to be more resilient against an invasion of non-beneficial bacteria, e.g., c. Difficile).
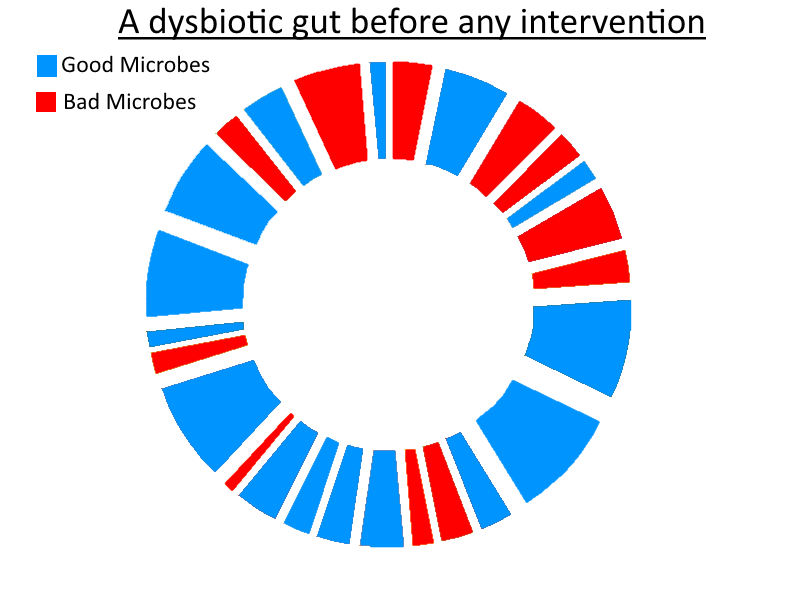
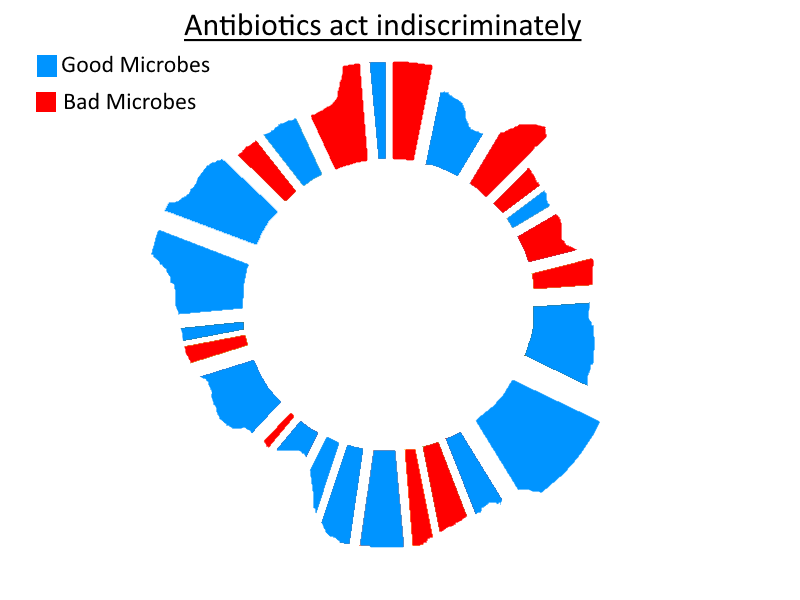
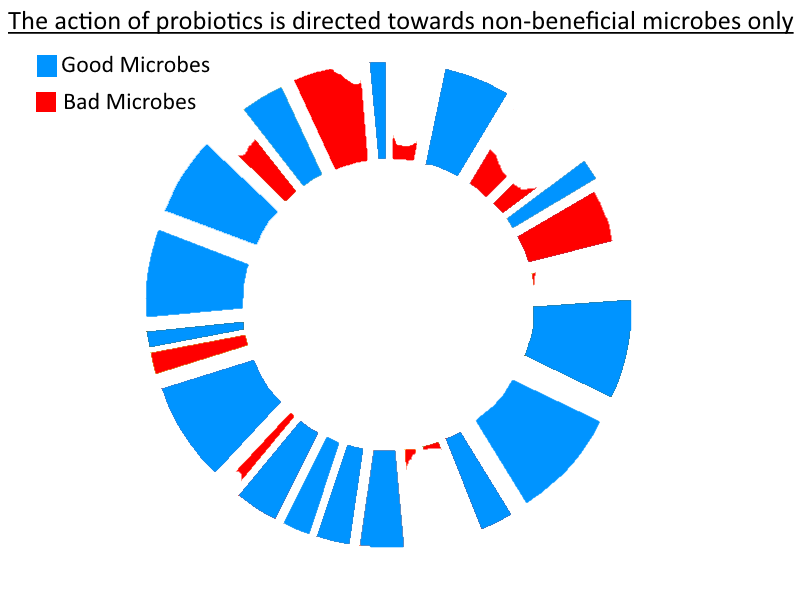
So – as stated in the title for my article on prebiotics/FODMAPs (credit to Richard Nikoley for the title!) – probiotics really can be seen as mercenaries. So if you thought that was only a play on the Uganda theme of the article (as I did when I first saw it!) consider the following similarities between probiotics and mercenaries:
- They are best utilised temporarily (at least that is my stance on probiotics).
- Mainly when there is a problem to resolve (i.e. not in times of peace/good gut health).
- They do not in any way represent the operational diversity of a fully functioning army (or in this case, a healthy microbiota).
- They come with weapons (chemical weapons – bacteriocins, fungicins, pH modulating byproducts, etc.).
- They can cause a tonne of disruption and evict a belligerent.
- And finally, they can be decommissioned once sufficient change has been made such that the regular microbiota can handle things from there on out.
Sticking with the analogy – some of these mercs may stick around to join the regular military (military = the vast ranging, self-sustaining, permanent gut flora in our large intestine), especially if some of them possess skills which were deficient in the regular army, i.e. they have an available niche to fill in a gut ecosystem (because, yes, you may well need an increase in Bifidobacterium Infantis, for example).
I didn’t always think this. A lot of it was due to the success that Elixa V1.0 had. I knew that version 1 was nowhere near representative of the full range of a healthy microbiota (in the way that, perhaps, an FMT is). It was still helping people immensely. That’s why I started to care less about specific strains and more about overarching modes of action at the species and genus level. For example, the ability to create lactic acid thus lowering pH. This was not a strain-specific mechanism. And perhaps it didn’t require a range of 5 Lactobacillus species to accomplish (although I am sure that there will be some variation between the bacteriocins that those 5 separate species produced and other differences in competitive exclusion mechanisms) – any Lactic Acid Bacteria could have done the trick.
It would not even surprise me if Elixa could generate a benefit in someone who already had those 12 species in abundance because you are still jacking up the overall chemical warfare going on in the gut. And that chemical warfare is targeted. Perhaps more so than any antibiotic could be. Take pH modulation for example. When lactic acid bacteria create d-lactate and lower the pH of the intestinal environment, this doesn’t just benefit themselves. A lower pH has been found to be optimal for beneficial bacteria and generally sub- optimal for non-beneficial bacteria.
It doesn’t take much to imagine that the bacteriocins which beneficial bacteria produce will selectively target only non-beneficial species. So the ‘targetedness’ probably spans across their entire armament. Whether the probiotic course causes a permanent or long-lasting increase in the quantity of those particular species in your microbiota will be dependent on whether there was a gap in that niche to begin with – and I do not think it’s even important. In fact, I would not be surprised if a ‘Lact/Bif’ probiotic, that caused significant resolution of a gut problem, left behind a substantial (and sustained) increase in an entirely different genus. One that may have previously been repressed by whichever set of bad microbes were overgrown in your gut. Said bad bacteria having now been evicted by the probiotic species.
Rock, paper, scissors
(with the twist that Rock has no conflict with Scissors)
Paper (a bad species) is well adapted to suppress Rock (a good species). Rock is poorly suited to counteract Paper.
Scissors (the species in the probiotic) comes along and gets rid of Paper with ease. Rock is free to flourish, now that Paper is gone.
Scissors leaves (i.e., the probiotic course ends)
Paper’s population is so diminished that Rock has no problem keeping its foothold now that it has been allowed to regain its former population size.
Net result:
- Paper has gone
- Rock flourishes
- No net change in Scissor population
Had it been a different bad species, Rock may have had no problem regaining its foothold by itself. Hence, why sometimes people bounce back easily from antibiotic-associated diarrhoea (for example).
The natural, healthy microbiota is so diverse that they will all be working together, handling different tasks that benefit each other. One species may breakdown substrate X and produce metabolic byproduct Y as a result. Another species may utilise byproduct Y but would not have been able to produce Y itself. Mutual co-existence.
So … probiotics as targeted antibiotics versus probiotics as deficiency solvers. Mull it over.
This last point is for another time, but I’ll briefly touch upon it. A separate mechanism by which probiotics can exert an effect involves a beneficial immune response to the presence of the probiotic bacteria – even if they are dead!
I mention this because I think it is the best explanation for why some people have reported skin improvements within 24-36 hours. I don’t see that the competitive exclusionary actions of probiotics could show a change that rapidly. So I would suspect it is some effect on the immune system which causes that lowering of inflammation in the skin resulting in improved texture and complexion (for some) to begin with, followed by additional benefits once the probiotic has had time to evict significant numbers of the bad microbes in your gut.
The next logical step is to give an ultra high potency probiotic a try! I’ve been developing Elixa over the past 5 years and it has experienced an unbelievably positive reception ever since I first launched it. I now sell to customers in over 30 countries, have fulfillment warehouses in 2 countries (UK & USA), and the demand is rising every day. I look forward to hearing great feedback from you, if you decide to try it for yourself. Just get in touch via email if you have any questions. I’ll be happy to help!


